Key Takeaways
- AOD-9604 is not FDA-approved and is available for research purposes only.
- Clinical data suggests modest weight loss and metabolic benefits in some patients.
- Patients may start noticing effects within 1 to 3 months, with varying results.
- Compliance with dosing and lifestyle factors significantly impact outcomes.
- Comparative outcomes with alternatives like growth hormone secretagogues vary.
What Is AOD-9604?
AOD-9604 is a synthetic peptide fragment derived from the human growth hormone, specifically amino acids 177–191. It is designed to modulate lipid and glucose metabolism pathways, primarily through β-adrenergic and AMPK-related mechanisms. While AOD-9604 is under investigation for potential metabolic benefits, it remains a research peptide and is not FDA-approved for clinical use. For more detailed information, visit the AOD-9604 full profile.
What Clinical Trials Show
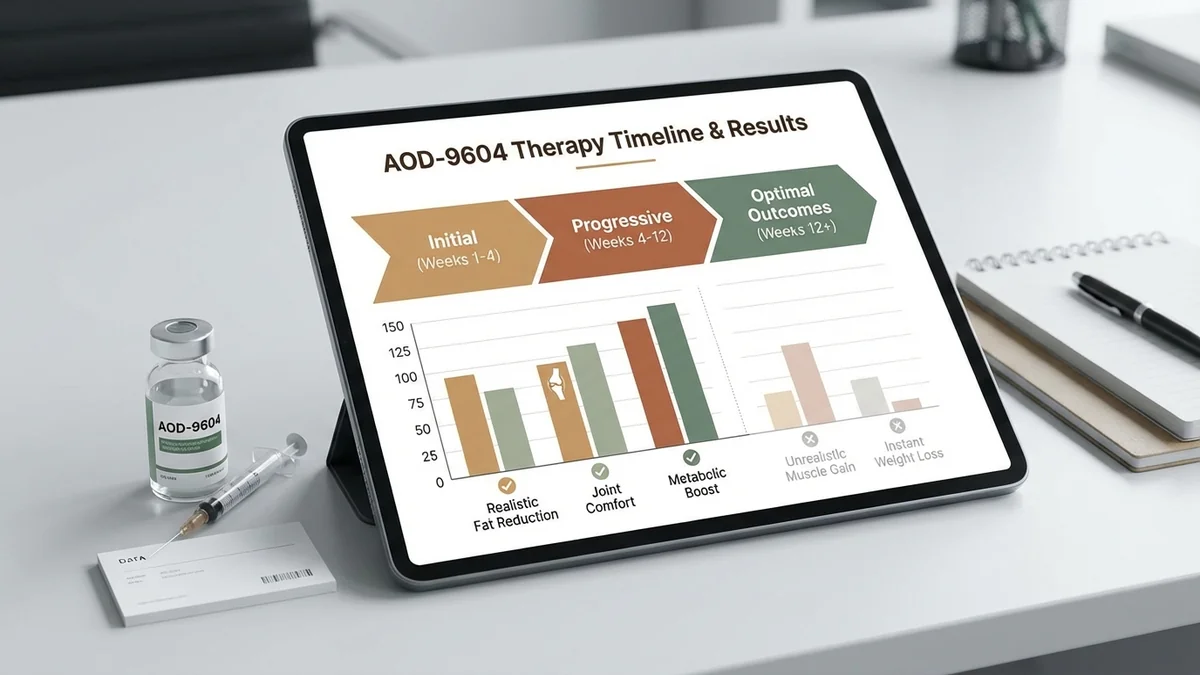
Clinical trials have primarily investigated AOD-9604's potential in obesity management. The Phase IIa trial (PMID 15134286) demonstrated that while AOD-9604 could promote weight loss, the results were modest. In this trial, participants experienced an average weight loss of 2.8% over 12 weeks, with around 30% of participants achieving this reduction in body mass. However, the trial highlighted that the peptide did not significantly alter the WADA hGH isoform immunoassay (PMID 24124033), indicating a distinct mechanism from standard growth hormone therapies.
Realistic Timeline
Patients might start noticing initial effects of AOD-9604 within 1 to 3 months. During the first month, subtle improvements in energy levels and metabolic rate might be reported. By the three-month mark, clinical data indicates that some patients may experience weight reduction and body composition changes. However, significant results are more likely to become apparent after consistent use over six months, aligning with findings from preclinical studies (PMID 41490200).
Factors That Affect Results
The effectiveness of AOD-9604 can be influenced by several factors:
- Dosing Compliance: Adhering to the recommended subcutaneous injection protocol is crucial. Variations in dose timing and frequency can impact outcomes.
- Diet and Exercise: A balanced diet and regular physical activity can enhance the peptide's metabolic effects.
- Underlying Conditions: Metabolic disorders or hormonal imbalances may affect peptide efficacy.
- Concurrent Medications: Other medications, especially those affecting metabolism, can interact with AOD-9604.
- Individual Variation: Genetic factors and lifestyle differences result in diverse responses among patients.
What Results Look Like in Practice
In practice, patients using AOD-9604 may experience mild to moderate improvements in body composition and metabolic health. Clinical data suggests that many users report an increase in energy levels and slight reductions in body fat percentage. However, these outcomes are not guaranteed, and individual experiences vary widely. Some patients may not notice significant changes, reflecting the need for combined lifestyle interventions.
Results Compared to Alternatives
When compared to other peptides and metabolic treatments, AOD-9604 generally offers more subtle results. Growth hormone secretagogues like ipamorelin and CJC-1295 often result in more pronounced changes due to their broader physiological effects. Non-peptide alternatives like GLP-1 agonists have demonstrated more significant weight loss in clinical trials (e.g., the STEP 1 trial, NCT03548935).
When AOD-9604 May Not Work
AOD-9604 may not be effective for everyone. Non-responders may include individuals with underlying metabolic conditions that are not addressed by the peptide's mechanism of action. Additionally, those seeking rapid or dramatic weight loss may find other treatments more suitable. Contraindications and individual health profiles should be assessed before considering AOD-9604.
What the Evidence Does Not Show
There is limited long-term data on the outcomes of AOD-9604 use. Its effects on diverse populations, such as those with severe obesity or metabolic syndrome, remain under-researched. Current studies primarily focus on short-term metabolic outcomes, leaving gaps in understanding its sustained efficacy and safety.
FAQ
How quickly can I expect to see results with AOD-9604?
Initial effects may be noticed within 1 to 3 months, with more pronounced outcomes potentially developing over six months.
What are common side effects of AOD-9604?
As AOD-9604 is still under research, comprehensive side effect data is limited. Mild side effects may include injection site reactions.
Is AOD-9604 better than other weight loss treatments?
AOD-9604 offers unique metabolic benefits but generally results in more modest weight loss compared to alternatives like GLP-1 agonists.
Can I use AOD-9604 with other medications?
Consult a healthcare provider before combining AOD-9604 with other medications, as interactions may affect its efficacy and safety.
What should I do if AOD-9604 doesn't work for me?
If AOD-9604 is ineffective, consider discussing alternative treatments with a healthcare provider.
Medical Disclaimer: This content is for informational purposes only and does not constitute medical advice. Consult a licensed healthcare provider before starting any treatment.
Find a Peptide Therapy Clinic Near You
Browse our directory of verified peptide therapy clinics across the United States. Compare providers, read reviews, and request a consultation.
PeptideClinicLocator.com does not provide medical advice. Always consult a qualified healthcare provider before starting any peptide therapy. Regulatory status may change.